Introduction
It is quite easy to demonstrate that electric current is not the moving of electric charges. The mandatory property of electric current is magnetic field surrounding it.
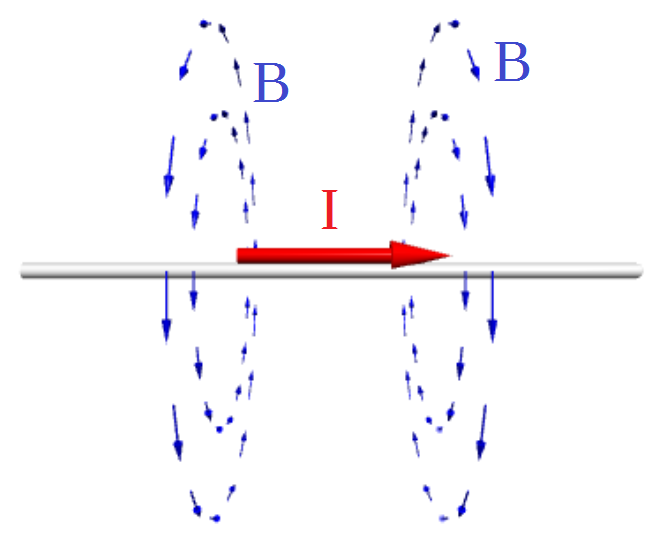
The problem is that magnetic field only appears when the charges are in motion. The observer could change coordinate system to the system moving along with electrons in the wire. In such system magnetic field produced by moving electrons disappeared. But physics teaches us that the physical processes should be the same in all moving systems. Here the trick – indeed electrons are stationary, but instead positively charged atoms are moving in the opposite direction along with the wire. As a result magnetic field remains the same.
Imagine now the beam of electrons. It is electric charges in motion, but is it electric current? Try to make the same trick with moving reference system. You find out that electron's magnetic field disappeared and there are no positive charges to maintain the field. The physics here contradicts to itself. On one hand the beam of electrons is electric current, but on the other hand the existence of magnetic field is highly questionable. It looks like electric current is not the moving of electric charges.
As we remember from school, the metals are good conductors of electric current. All metals are full of free electrons, which makes the current flow possible.
Now we suspect that the electric current is not what it seems to be, it is not moving of charges and we don't need free electrons conjecture anymore. Are free electrons in metals real? Let's take a closer look.
Free Electrons
The only reason for existence of free electrons in metals is the fact that electric current defined as charge in motion. In current concept of physics electrons are orbiting around nucleus. There is some negative potential energy attributed to that motion, which mean that we need to spend some energy in order to separate electron from nucleus.
This energy, also called ionization energy, could be found in technical data for elements. For Copper ionization energy equals to 745.5 kJ/mol.
It is quite difficult for ordinary people to deal with moles, which is measure for quantity of substance and better to recalculate this value as energy per kilogram. For Copper one mole is equal to 63.55 grams and ionization energy will be 11730.9 kJ/kg.
This energy also could be calculated from Bohr's model of atom. The result will be approximately the same.
How Big Is This Energy?
Now it is time to compare this ionization energy with something we could understand. Is it big or small?
In everydays life the energy supplied to the piece of substance will heat this substance by a certain amount of temperature. The formula describing the process of heating is:
$$\Large Δ Q = CM Δ T $$Where \(Δ Q\) is the heat supplied to the body, \(C\) is specific heat capacity of the substance, \(M\) is the mass of the substance and \(ΔT\) is temperature rise. In terms of temperature rise:
$$\Large Δ T = {{Δ Q} \over {CM}} $$From textbooks we could find that specific heat capacity of Copper equals to 384.4 J/kg/K. The mass of the Copper is one kilogram and:
$$\Large Δ T = {{11730900} \over {384.4}} \approx 30500 $$Wow! The energy required to make all electrons free (one per atom) in the piece of Copper is equivalent to heating same piece of Copper by THIRTY THOUSAND degrees!
Of course, the result is approximate and could be used just for the sake of comparison. The Copper will melt and evaporate in the process of heating and specific heats of liquid and gaseous states are different.
Within the single atom of Copper the electrons are bonded with nucleus. When single atoms of Copper were combined into solid piece, where this tremendous energy required to free electrons came from?
Electrical Resistance
It may sound strange, but another proof of the absence of free electrons in metals is the electrical resistance.
The most implausible fact about electrical resistance is that resistance is inversely proportional to the cross area of conductor. As a proof they teaching us that electricity is the same thing as water in the pipe. And water indeed experiencing bigger resistance in the pipes of smaller diameter.
Water analogy seems reasonable until you start thinking. What is the source of water resistance in the pipe? Talking about laminar flow, such source is the walls of the pipe. Resistance of the pipe is defined by surface area of the wall and the volume of water able to pass through pipe is proportional to the volume of pipe. The thinner the pipe the less ratio of the volume to surface is.
Talking about water analogy, better talk about pipe filled with fine sand. The resistance in such pipe will be proportional to the cross section of the pipe and the value of resistance will not be depend on diameter.
What is the source of electrical resistance? Is it the surface of conductor similar to water analogy? Completely not! As per textbooks, the source of resistance is interaction of electrons crystal lattice of metals. So the “volume” of electricity is proportional to the volume of wire and so the resistance is also proportional to the same thing. Resistance should not depend of the cross section!
The following explanation could often be found in the literature: the bigger cross section has bigger number of free electrons, which means that current flow increases. Here is the example why such claim just not true. Well known fact that all electrons from outer shell became free in metals. The following metal are very close to each other: Chromium (Cr) and Manganese (Mn). Atomic weights are 52 for Cr and 55 for Mn. Densities are 7.19 g/cm3 for Cr and 7.47 g/cm3 for Mn. With atomic weight and density known, we could calculate interatomic distance for any material. The interatomic distance for Cr is 2.29e-10 and 2.3e-10 for Mn. Huge difference between metals is the fact that Chromium has one electron in outer shell while Manganese has two. From above explanation the resistance of Manganese should be twice less compare to Chromium. In fact the resistance of Manganese is twelve times bigger!
The existing of “free” electrons in metals are simply not possible.