Introduction
Black Body Radiation is a complete mystery. Planck's law is a mathematical formulation of electromagnetic radiation emitted by a black body in thermal equilibrium. Astronomers calculates star's temperature by measuring its spectrum and finding the peak on the radiation curve. Looks straightforward until you realize that two forms of Planck's law exist – energy density as a function of wavelength and energy density as a function of frequency. Those two curves produce dramatically different results. The temperature of the Sun is around 5760K as follows from wavelength curve, but at the same time the temperature of the Sun is around 10000K as follows from frequency curve.
But such a fact is not a mystery, it is just a feature of modern science, the mystery is the complete absence of physical mechanism. The body could not emit radiation just because mathematical formula exists, it emit due to some physical mechanism, and we just don’t know it yet.
Background
This days everybody familiar with radiation spectrum, especially from climate science. Here for example is spectrum curve for the energy radiated by a body at near room temperature of 300K.
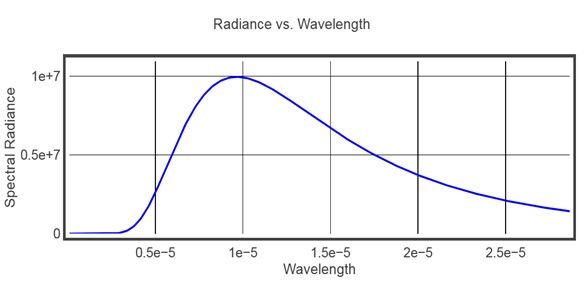
Fig.1 Black body radiation spectrum at 300K.
The maximum of the curve located approximately at 9.65 microns.
Kinetic Theory of Gases
The ideal gas follows Maxwell-Boltzmann distribution of molecular motion. The average kinetic energy of the molecules of monoatomic gas could be defined as:
$$\Large { {1 \over 2} m \overline{v^2} = {3 \over 2} k_B T \tag{1} } $$Where \( k_B \) is Boltzmann constant and \(T\) is temperature.
Let our emitting body be Helium gas at temperature of 300K and pressure of 1 bar (105 Pa).
Then the average kinetic energy of molecule according to (1) will be:
$$\Large {1 \over 2} m \overline{v^2} = 1.5 * 1.38 \cdot {10}^{-23} * 300 = 6.21 \cdot {10}^{-21} \tag{2} $$Radiation
According to quantum mechanics our gas should emit electromagnetic radiation. The maximum energy of such radiation will be located at wavelength of 9.65 microns (at 300K temperature).
The electromagnetic radiation could not be just emitted. It could only be emitted in quanta or photons, which are the minimal portion of energy.
The energy of photon is known from quantum physics and equals to:
$$\Large E = h \nu \tag{3} $$Where \(h\) is Planck constant and \( \nu \) is frequency.
Frequency and wavelength are connected by the following equation:
$$\Large c = \lambda \nu \tag{4} $$Energy of one photon with wavelength of 9.65 microns will be:
$$\Large E = 6.53 \cdot {10}^{-34} * 3 \cdot {10}^{8} * 9.65 \cdot {10}^{-6} = 2.06 \cdot {10}^{-20} \tag{5} $$Ooops! The energy of photon is 3 times bigger compare to average kinetic energy of molecules. Not a good start.
It is also obvious that the molecule of gas could not emit photon during its straight motion without acceleration. It could only emit something during collision.
Collisions
The situation is even worse. The molecules of gas could not give up all of its kinetic energy at once. The collisions must be elastic, only a tiny amount of kinetic energy could be emitted as radiation. If collisions of the molecules are not elastic, then the whole kinetic theory of gases is wrong, Maxwell-Boltzmann distribution is wrong and so on.
But it’s not only that. The collision of molecules not always happened right in the forehead.
Fig.2 Forehead collision of gas molecules.
More likely it will be something like this:
Fig.3 Sideway collision of gas molecules.
And even smaller part of kinetic energy could be utilized as radiation.
Inelastic Collisions
Let's consider one dimensional motion of two bodies (forehead collision). If collision is completely inelastic then kinetic energy is not conserved. In the case of macro objects, the energy difference converted to a temperature change. In the case of collision between two molecules there is no such thing as temperature and where the energy goes? Probably into radiation, but don't try to find the answer in the textbooks.
After inelastic collision two body “glued” together and continue moving as one body. The momentum is conserved:
$$\Large m_1 v_1 + m_2 v_2 = ( m_1 + m_2 )u \tag{6} $$The difference in kinetic energies before and after the collision:
$$\Large \Delta E = {1 \over 2} m_1 v_1^2 + {1 \over 2} m_2 v_2^2 - {1 \over 2} (m_1 +m_2)u = {1 \over 2}{{m_1 m_2} \over {m_1 + m_2}} {(v_1 - v_2)}^2 \tag{7} $$And the energy available for radiation is proportional to the difference in velocities squared.
In the case of 3D motion of molecules, the velocities in (7) are not absolute velocities of molecules, but instead we should use projection of said velocities to the vector connecting two molecules. They are velocities toward each other.
As was mentioned before, collisions could not be fully inelastic, but radiated energy should be in proportion to mutual kinetic energy of two particles toward each other (7).
Distribution
Radiation energy should be proportional to mutual kinetic energy of two molecules during collision.
The distribution of this relative energy should be very similar to Planck energy distribution. In order to study the subject, the gas simulation program from Bruce Sherwood was modified to save some amount of collision data such as position and velocity vectors.
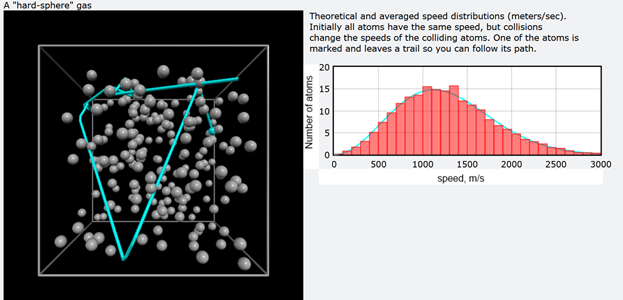
Fig.4 Ideal gas collision simulation.
The mutual kinetic energy of collisions was calculated and distribution of such energies is shown below:
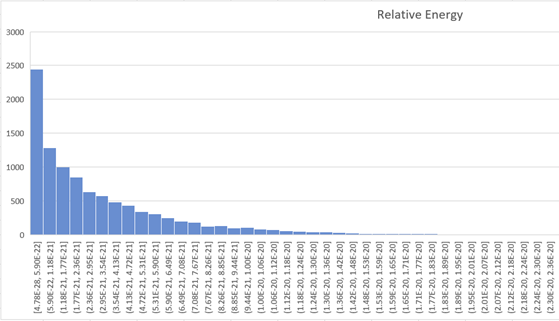
Fig.2 Energy distribution of molecular collisisions.
There are two things to mention. First, distribution of relative energy is not even close to the Planck's distribution and the possible mechanism of radiation most likely is not connected to the collision of molecules. If straight motion of molecules does not produces radiation and collision of molecules does not produces radiation, what does?
Secondly, most of the collision energies are below our estimation for average kinetic energy of molecules (2). And the real radiation energy should be significantly less in order to keep gas behavior similar to ideal gas.
There is no energy in the gas available for radiation of photon. It is not logical to guess that radiation of single photon produces by multiple collision of many molecules. Also low energy photons should prevail in radiation, which contradicts to Planck's distribution.
Conclusion
Black Body Radiation contradicts to the quantum nature of radiation. It was also demonstrated that Black Body Radiation could not originate from the collision between molecules of gas.