Introduction.
Following experiment conducted in order to study latent heat phenomenon. Experimental data analysis did not shows any sign of latent heat.
Setup.
The following picture shows setup of experiment:
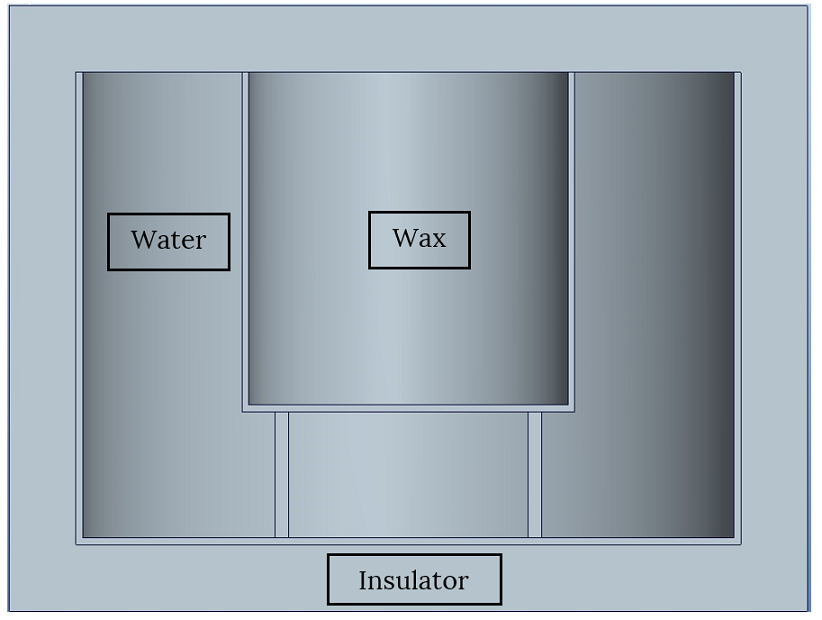
Internal metal vessel filled with melted wax was placed inside another vessel, containing water. The outer water vessel was surrounded with insulation preventing heat from going outside. Temperature of water and wax was monitored using thermocouples until all wax crystallized. The amount of wax was approximately 350 ml and the amount of water was approximately 500ml.
Data.
Collected data show on the graph below:
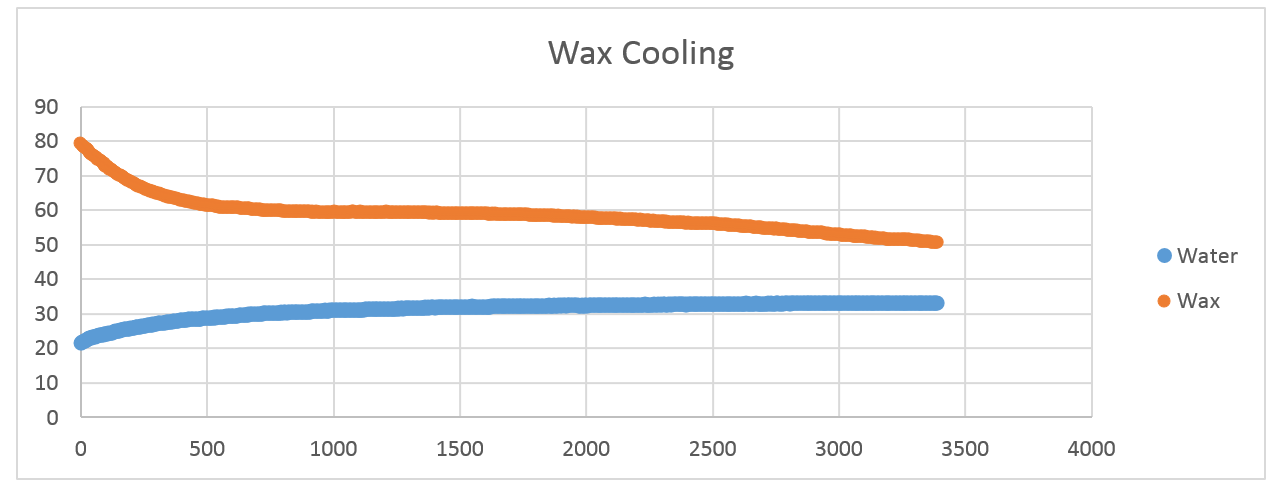
The question is if significant amount of heat was released during wax crystallization, then where it went?
The amount of latent heat should be tremendous. Here the rough estimation: heat of fusion for wax is approximately 240 kJ/kg and heat released Q = 240*0.35 = 84 kJ. There is formula, provided by thermodynamics: Q = C*M*ΔT. Specific heat of water equals to 4.186 kJ/kg/K and ΔT = 84kJ/0.5kg/4.186 = 40C. Such heating by 40 degrees was not observed. Where this heat go? Must be “tunneling” to the ambient without even touching the water.
Moreover, the water temperature approximately follows exponential function similar to Newton’s law of cooling. In fact, it is something like this:
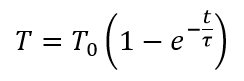
The water temperature curve was analyzed against such dependency using least square method. Analysis were done for three independent periods of time – when wax was liquid, crystallization period and solid wax period. Analysis shows that the water temperature indeed follows above formula and the formula is the same for all three zones. It shows that there was no additional heat income during wax crystallization period.
Conclusion.
According to experiment, latent heat of fusion does not exist. The absence of latent heat automatically lead to non-conservation of energy during phase transition. Probably the energy conservation law still holds if we redefine the heat and the temperature. All thermodynamics – heat, energy, temperature become a house of cards.