Introduction.
Everyone familiar with latent heat phenomenon. Ice's temperature rising during heating. Everything very clear and physics is in a good position – you provide energy income and the temperature goes up. However, at some point melting temperature reached. The energy input still there, but temperature stays until all ice melts. Energy conservation law does not holds here. In order to save face, “latent heat” term appeared in 1762 due to Joseph Black's discovery. There are very large number of phases in solids, transition between ice VI and ice VII, for example. For the purpose of current article, whenever latent heat appeared, it means latent heat of melting or solid to liquid transition.
Temperature.
The phenomenon of temperature still waiting for investigation. While there are some physical models of temperature for gases, like kinetic theory, the solids heating and cooling remain unexplained. Looks like the science did not moved anywhere since ancient Greek times. Only blah-blah-blah stuff exist in the scientific literature, like the animation of vibrating molecules. Not even one formula available for the solids. Do vibrations really exist in solids?
Solids.
Physics telling us that solid is one of the fundamental state of matter. Be careful, when science use word “fundamental”, it automatically mean – “without any explanation”. There are four types of solids according to modern scientific classification of intermolecular bonds:
• Ionic bonds, NaCl
• Hydrogen bonds, H2
• Metallic bonds, metals
• Covalent bonds, diamond
Some formulas exist only for ionic bonding, everything else are just on descriptive level. Ionic bonding is just Coulomb force between charged particles, which is in inverse proportion to the square of distance.
Coulomb Force.
I am going to focus on solids with ionic bonding, since they are most described solids. The forces inversely proportional to the distance squared are not physical. Such forces are gravitational and electrostatic.
The orbits under such forces could not be stable. Indeed, when you look at the stone circling overhead at the end of rope, you will find that the orbit is extremely stable. If the radius increased due to some fluctuation, then the force from the rope will also increase, returning stone to the previous orbit. We have the system with negative feedback. The force is in proportion to the displacement and have the opposite sign.
The orbiting under influence of gravitational and electrostatic forces are something completely different. Increasing the radius, we have reduction of attractive force and there is absolutely no returning force, stabilizing the orbit. Such fact is difficult to observe on astronomic scale with its millions of years timescale, but in micro world with billions of collisions per second the existing of such orbits are simply not possible.
Now we are getting close to most interesting part – the vibrations. The formula for the one-dimensional motion of the particle in crystal lattice under Coulomb force will look like:
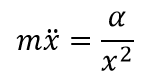
The solution of such differential equation is aperiodic function. In other words, no vibrations are even possible. And this is exact reason why no formulas could be found in the literature – it simply could not exist.
So, the situation is as following – either temperature of solids should be redefined or intermolecular forces in solids are something very different from Coulomb forces.
Intermolecular bonds.
Latent heat in modern interpretation is the energy required to break the bonds between molecules of solids. Do the bonds really store the energy?
Physics deals with models. We should build some model of intermolecular bonds in order to discuss anything further. Do you remember your chemistry lessons in school with stick and balls models? In order to make thermal vibrations possible we replacing sticks with a springs, good enough.
Now the springs connected molecules together and allow them to vibrate at the same time. Everybody agree on the fact that the spring could contain some potential energy, but do such bonding itself possess energy? Not really. For the sake of simplicity, let us look on just diatomic crystal. The words “kinetic vibration energy” are useless. Kinetic energy of vibration is not constant. Kinetic energy of atoms at the maximum speed transferred into potential energy of the spring at zero speed. Kinetic to potential and vice versa, back and forth. By the analogy with harmonic oscillator, we could only speak about total energy of oscillator or the kinetic energy when the speed is maximum.
Consider two molecules heading to each other with some speed. What would be the temperature of such system? Right – average kinetic energy. Now we are connecting those two moving molecules with the spring at some point, forming a solid body. Instead of linear motion, there are oscillations. What would be the energy of oscillations? The total energy of our oscillator will be equals to the kinetic energy of molecule’s linear motion prior to spring connection. No additional energy could be stored in spring bonding.
Our next experiment on newly invented bond will be the fusion. Let’s heat our solid consisting from two molecules and the spring. Heating will result in increasing the amplitude of oscillation (temperature increase) until the weak point of the spring reached. It is obvious that spring will break at the maximum elongation, when potential energy is maximum, leaving our two molecules fly away with near zero speed and hence the temperature.
Definitely, our vibratory model does not work in the way observed in nature. Something is wrong, either model or nature.
The Facts.
Physics is the science of experimenting. Let us take a look at experimental data. It is well-known experiment on supercooled water.
Distilled water in the fridge could reach below freezing temperature staying in liquid state. When the bottle removed from the fridge and shakes, the ice will formed through the volume of water. There are a lot of videos on Youtube.
We could make the following assumptions – the mass of the bottle was one kilogram and the temperature in the fridge was -10C.
Freezing ice should release the heat of fusion, otherwise the energy is not conserved.
Latent heat of fusion for water equals to 334 joules per gram and total released energy equals to 334000 joules.
Now I am going to calculate the increase of the temperature of the ice due to such amount of energy. The heat capacity of ice equals to 2.108 kJ/kg/K and temperature increase equals to 334k/2.108k = 158 degrees! Obviously, such amount of temperature increase was not observed. It is amazing that such simple experiment completely ignored by modern science.
Oh, sorry, I forgot to spend some energy on the water heating from zero to -10C. The heat capacity of water is two times more compare to ice – 4.184 kJ/kg/K. Energy, required for heating the water will be: 4.184*10 = 41.84. Now we have less energy for ice heating: 334k – 41.84k = 282k. New temperature increase will be 138C!
Conclusion.
According to experiment, latent heat of fusion could not exist. The whole theory of temperature, heat, phase transition and constitution of matter still waiting for researchers.