Introduction.
Everything strange at low temperatures. The properties of matter, especially electric and magnetic properties undergo great changes at such extreme condition. Superconductivity, superfluidity, various magnetic phenomenon – all these effects are very different from what we see every day at room temperature.
We were taught that there is no activity at extreme low temperatures, everything freezes and stop moving. Even entropy is zero at zero temperature. But…
Electrons.
Are electrons stop moving at low temperatures and fell to the nuclei? If so, why they did not annihilate with protons? Or annihilation is possible with positron only? Much more questions than answers. Here I am going to make some estimation related to the electrons motion at low temperatures.
Temperature.
The temperature of the monoatomic gas is in proportion to the average kinetic energy of the molecules:
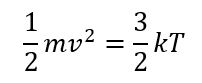
Let's go with Helium gas at the temperature close to the liquid form of Helium (4K). After calculations we got the following values:
• Average energy of molecules - 8.3∙10-23 Joules
• Average speed of molecules – l58 m/sec
Electrons Math.
The orbiting radius of electron could be found in Bohr's atomic model. Although the modern science getting away from Bohr's model to something like electron cloud, the size of such cloud is very close to the Bohr's one. Bohr's radius could be calculated using formula:
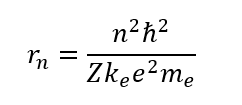
Which gives us for the Helium atom 2.65∙10-11 m.
The speed of orbiting electron could be calculated using the balance between centripetal and Coulomb force:
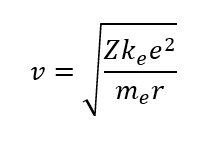
And the speed equals to 4.36∙106 m/sec. A little bit too far for freezing!
The energy of single orbiting electron would be 8.68∙10-18 Joules. Five orders of magnitude bigger than the energy of nucleus! And we have two electrons. It is obvious that all properties of Helium at low temperature should be refer solely to the orbiting electron.
The “temperature” of such electron will also be five order of magnitude bigger or 400,000K! Good chance for nuclear fusion!
Our next step will be cooling down the rotating electron to 4K. Then the energy of electron will be equal to the one of nuclei and the speed will be 1.35∙104 m/sec.
Using above formula for balancing centripetal and Coulomb force we could find that “frozen” orbiting radius should be 2.78∙10-6 meters! It is close to 3 microns, while optical microscopes could resolve distances down to 0.2 microns. With nuclear radius around 10-15m such radius looks like electrons are shared in low temperature Helium like in metals.
Besides there are no known mechanisms of transferring “electron's temperature” to “nuclei temperature”, aren't they? Such mechanisms just could not exist from logical point of view. It is quite easy to say that centripetal force is balanced by Coulomb force and just use math for finding the radius. From physics point of view imagine electron which already in rotation with some energy and radius corresponding to room temperature. The system is cooling down and some miraculous mechanism decrease the energy and the speed of orbiting electron. The centripetal force decreases, and Coulomb force will push electron close to the nuclei, instead of freshly calculated bigger radius.
And yet they move?