Introduction
The term “temperature” was always a riddle for me. Definition from Wikipedia as “physical quantity expressing hot and cold“ is nothing more than circular. Indeed, the hot means having higher temperature. Here I tried putting together different approaches to the temperature definition.
Entropy Approach.
Temperature defined as the ratio between the heats added to the system and the increase of entropy.
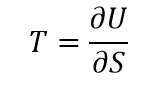
Such definition does not look very scientific, because it requires some additional quantity “entropy”, which could not be directly measured. It is also circular, because the term “entropy” had introduced in 1860s by Clausius as a ratio of heat to temperature. The evolution of entropy is a subject of separate study.
Very often, the notion “entropy” presented to the students by the example of colored balls. How do you know that nature is not colorblind? And vice versa – some really important properties of those balls could be well above humans perceptions.
Kinetic Theory Approach.
Kinetic theory is an attempt to introduce some physics in the definition of temperature. Kinetic theory describes gas as a large number of submicroscopic particles, all of which are in constant and random motion. Any search on the internet for “kinetic theory” will provides only one result – “kinetic theory of gases”. Kinetic theories of liquids and solids do not exist.
Anyway, let's take a look at gases. The theory claims that temperature is equals to the average kinetic energy of the molecules with some scaling factor.
The physics with absolute motion gone long time ago. There is no place for absolute motion in modern physics. The motion of a body could only be observed by attaching a frame of reference to an observer and measure the change in body's position.
This rise an interesting question – what the speed of molecules are relative to? The question stays unanswered in physics textbooks.
Relative character of kinetic energy makes the temperature also a relative value. If temperature is the parameter of the body depending on the choice of reference frame, then common sense should dictate that such reference frame should be one associated with thermometer.
On the other hand, it is possible to measure temperature without thermometer, using black body radiation formula, which is not velocity dependent. Should we change the formula to a velocity dependent one? Should such velocity dependent formula includes Doppler effect?
The variation of temperature due to relative character of the motion is very small at normal conditions. Although such variations are extremely big at supersonic speeds. Is it a source for aerodynamic tension? For example, the average speed of air molecules at room temperature is about 650 meters/sec. Supersonic speed equals to three Mach numbers is approximately 1000 m/sec.
Temperature definition also fails when used to describe phase transition. I don’t want to mix up here, phase transitions and latent heat will be discussed in separate article.